Introduction
The CART-SIE is a national prospective/retrospective observational study aimed at collecting the real-life data of all consecutive lymphoma patients treated with CAR-T cells in the Italian centers. Given the recognized efficacy of axicabtagene ciloleucel (axi-cel) and tisagenlecleucel (tisa-cel) in relapsed/refractory large B-cell lymphoma (R/R LBCL) after at least two previous treatments, the best product in terms of efficacy and safety is still a matter of debate. Riedell reported comparable outcomes between axi-cel and tisa-cel in patients treated in 8 US centers; on the other hand, Bachy demonstrated, in a matched comparison, that axi-cel had a higher efficacy and a higher toxicity compared to tisa-cel.
Methods
On these bases, we conducted a subgroup analysis in the CART-SIE study, with the aim to evaluate the outcome (ORR, DoR, OS, PFS), and the safety (CRS, ICANS) of all R/R LBCL treated with different CAR-T (axi-cel versus tisa-cel) (primary mediastinal B-cell lymphoma, PMBCL, were excluded). A propensity score (PS) model estimated for the probability of being treated with tisa-cel (arbitrary) was performed. Variables used for the PS model (accordingly to Bachy, 2022) were: histology, age, sex, disease status (relapse vs. refractory), Ann Arbor stage (I/II vs. III/IV), IPI (< 3 vs. >=3), LDH, C reactive protein, bulky disease, N of previous treatments, ASCT, bridging therapy (no vs. yes with response vs. yes without response), time since last treatment and centers size (>=25 vs. < 25 cases contributed).
Results
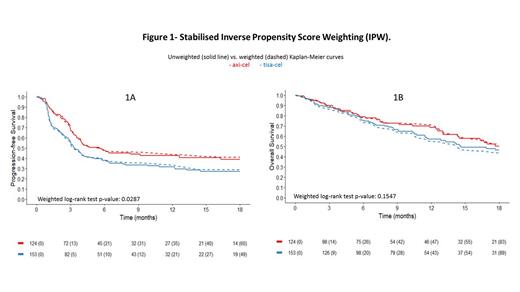
From March 2019 to June 2023, 659 patients were leukapheresed; 562 were infused and 556 with adequate follow-up were analyzed: 419 LBCL (229 diffuse large B-cell lymphomas, DLBCL; 77 arising from indolent lymphoma, tFL; 113 high-grade B-cell, HGBCL), 70 PMBCL and 67 mantle cell lymphomas. Here, we analyzed the results of the 419 LBCL: 45% (190) received axi-cel, 55% (229) tisa-cel. Clinical characteristics for axi-cel vs. tisa-cel were: median age 57 years (IQR 50;65) vs. 61 (IQR 52;66), p 0.059; refractory to the last treatment 74% (141) vs. 64% (146), p 0.0309; intermediate-high/high risk IPI 39% (74) vs. 46% (105), p 0.1336. Bridging therapy was performed in 82% (156) patients candidate to receive axi-cel and in 85% (195) tisa-cel, p 0.4263; the ORR to bridging was 28% (53) in patients candidate to axi-cel vs. 30% (68) tisa-cel, p 0.1840. All grade CRS was observed in 88% (168) infused with axi-cel, and in 77% (177) with tisa-cel, p 0.0030. The severe (grade 3-4) CRS was registered in 8% (15) axi-cel and in 8% (18) tisa-cel. All grade ICANS was recorded in 32% (61) treated with axi-cel and in 9% (20) with tisa-cel, p 0.0001; severe ICANS was reported in 9% (18) axi-cel and in 3% (6) tisa-cel. Treatment related mortality was 2% (4) in axi-cel vs. 1% (3) tisa-cel. Median follow-up time for infused patients was 12.07 months (IQR: 6.05, 22.37). The ORR at 30-days after CAR-T was 74% (141) with 53% complete response (CR) in axi-cel; ORR 58% (133) with 39% CR in tisa-cel, p 0.001. DoR at 12-months was 56% (95% CI: 48-67) for axi-cel vs. 49% (95% CI: 40-59) for tisa-cel, p 0.2745. The 12-months OS was 71% (95% CI: 64-79) for axi-cel vs. 60% (95% CI: 53-67) for tisa-cel, p 0.1086. The 12-months PFS was 48% (95% CI: 41-57) for axi-cel vs. 32% (95% CI: 26-39) for tisa-cel, p 0.0003. In a multivariable model with all the clinically relevant variables, the OS for the two products was similar (HR tisa vs. axi: 1.23, 95% CI:0.86-1.75, p 0.2546), whereas the PFS was statistically superior for axi-cel compared to tisa-cel (HR: 1.65, 95% CI: 1.25-2.17, p 0.0004). The stabilized inverse propensity score weighting was applied to the subset of 277 LBCL (DLBCL, HGBCL, tFL) patients with complete data. The final population for weighted analysis included 201 patients, 100 treated with axi-cel and 101 with tisa-cel. The propensity score weighted analysis for tisa-cel vs. axi-cel was calculated; weighted log-rank test p-value for PFS was 0.0287 (Fig 1A), weighted log-rank test p-value for OS was 0.1547 (Fig 1B).
Conclusions
In the CART-SIE study, the outcome of patients treated with CAR-T was similar to those reported by pivotal trials and real-life studies, with manageable toxicities. At a median follow-up of 12 months, PFS for axi-cel was superior compared to PFS for tisa-cel; this advantage was confirmed in a propensity score analysis. The difference in PFS did not translate in a different OS, so far. CRS and ICANS were higher with axi-cel.
Disclosures
Chiappella:Celgene-BMS: Other: lecture fee/educational activities, advisory board; SecuraBIO: Other: advisory board; Novartis: Other: lecture fee/educational activities; Jannsen-Cilag: Other: lecture fee/educational activities, advisory board; Incyte: Other: lecture fee/educational activities; AstraZeneca: Other: lecture fee/educational activities; Takeda: Other: lecture fee/educational activities, advisory board; Ideogen: Other: advisory board; Roche: Other: lecture fee/educational activities, advisory board; Gilead-Sciences: Other: lecture fee/educational activities, advisory board. Casadei:Takeda: Membership on an entity's Board of Directors or advisory committees; Beigene: Membership on an entity's Board of Directors or advisory committees; Celgene-BMS: Membership on an entity's Board of Directors or advisory committees; Roche: Speakers Bureau; Lilly: Speakers Bureau; Novartis: Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Kite-Gilead: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Di Rocco:Roche: Honoraria, Speakers Bureau; Novartis: Speakers Bureau; Gilead: Honoraria, Speakers Bureau; Janssen: Honoraria; Abbvie: Honoraria; Takeda: Speakers Bureau; Incyte: Speakers Bureau. Botto:Takeda: Speakers Bureau. Ladetto:Novartis: Honoraria. Cavallo:Roche: Honoraria, Speakers Bureau; Takeda: Research Funding; Astra Zeneca: Research Funding; Beigene: Research Funding. Zinzani:GILEAD: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SECURA BIO: Membership on an entity's Board of Directors or advisory committees; CELLTRION: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BEIGENE: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; JANSSEN-CILAG: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SANDOZ: Membership on an entity's Board of Directors or advisory committees; MSD: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SERVIER: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ASTRAZENECA: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; EUSAPHARMA: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ROCHE: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; INCYTE: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; TAKEDA: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; KYOWA KIRIN: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; NOVARTIS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ADC THERAPEUTICS: Membership on an entity's Board of Directors or advisory committees. Corradini:AbbVie: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; ADC Theraputics (DSMB): Other: Honoraria (Consulting, advisory role, or lecturer); Amgen: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; Celgene: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; Daiichi Sankyo: Other: Honoraria (Consulting, advisory role, or lecturer); Gilead/Kite: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; Incyte: Other: Honoraria (Consulting, advisory role, or lecturer); Kyowa Kirin: Other: Honoraria (Consulting, advisory role, or lecturer); Nerviano Medical Science: Other: Honoraria (Consulting, advisory role, or lecturer); Janssen: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; Novartis: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; Pfizer: Other: Honoraria (Consulting, advisory role, or lecturer); Roche: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; Sanofi: Other: Honoraria (Consulting, advisory role, or lecturer); SOBI: Other: Honoraria (Consulting, advisory role, or lecturer); Takeda: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; GlaxoSmithKline: Other: Honoraria (Consulting, advisory role, or lecturer); BeiGene: Honoraria; Bristol Myers Squibb: Other: Travel and accomodations.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal